Medical Countermeasures
for radiation exposure and contamination
for radiation exposure and contamination
Video 1: Introduction
Read Transcript, Download (.mov, 56MB)
During a radiation emergency there will be a need to triage, assess, and treat patients who have come in contact with radioactive materials or who have been exposed to a high dose of ionizing radiation.
This training will introduce you to:
Video 2: Radiation Concepts for Medical Countermeasures
Read Transcript, Download (.mov, 41MB)
There are important differences between radiation exposure and contamination with radioactive material. An understanding of each is critical for effectively triaging and managing patients.
This section provides an overview of radiation concepts:
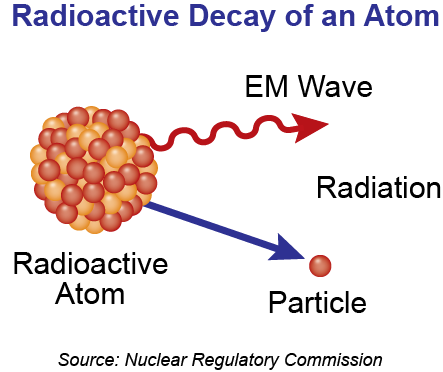
Figure 1: Radioactive decay of an atom
Radiation is a type of energy, or electromagnetic wave, ranging from low energy forms (e.g., radio waves) to high energy forms (e.g., gamma rays). Some high energy forms of radiation can ionize atoms by removing electrons (Figure 2). Ionizing radiation can therefore cause health effects and injuries by damaging biological molecules like DNA, proteins, or cell walls.
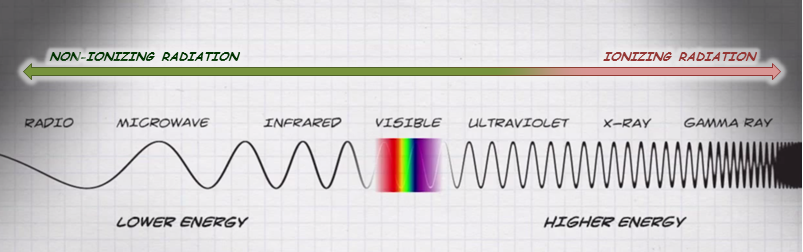
Figure 2: Electromagnetic spectrum – highlighting ionizing radiation
In this course, the term “radiation” will be used in reference to ionizing radiation. Types of ionizing radiation include:
Radiation exposure occurs when a person comes in contact with ionizing radiation. For example, a patient is exposed to radiation when receiving a chest x-ray.
Radioactive contamination occurs when radioactive materials are deposited either on or in the body. For example, if a bomb containing radioactive material explodes, people in the surrounding area could be contaminated with radioactive material.
The important difference between exposure and contamination is that an exposed person has received a one-time dose of radiation, while the contaminated person is being exposed to radiation and potential cell damage for as long as the contamination remains on or in them.
There are two types of radioactive contamination:
When a person becomes internally contaminated, the distribution of the radioactive material in the body will depend on the chemical form and particulate size of the material.
Radioactive material is removed naturally from the body through a combination of two processes - radioactive decay and biological excretion.
Medical countermeasures work by blocking the incorporation of radionuclides into tissues or by binding to the material so that it is excreted more rapidly. When radioactive material is excreted in a person’s urine or feces, that material is considered contaminated and should be handled using the appropriate precautions when it is transported, tested, or disposed.
Other countermeasures work by supporting the regeneration of cells damaged by high doses of radiation.
The following factors may limit the use of medical countermeasures:
More information can be found in the additional resources section below.
Video 3: Triage and Medical Countermeasures
Read Transcript, Download (.mov, 64MB)
During a radiation incident involving mass casualties, medical history and triage will be critical parts of an effective response effort. Clinicians and public health officials will need to work together to rapidly determine who receives medical countermeasures, which ones, when, and where.
Whenever possible, clinicians should consult radiation health professionals for guidance on radiation detection and dose assessment.
Effective triage during a radiation incident will include:
During a radiation emergency, individuals may present for care in multiple ways.
| Off-site Staging Areas | Emergency Arrival | Hospital Walk-in |
|---|---|---|
 |
 |
Source: REAC/TS |
Care providers may be afraid to treat patients due to the presence of radioactive contamination, but real-world experience suggests there is minimal risk to healthcare providers and staff when appropriate protective equipment, such as standard precautions, is worn.
The need for medical countermeasures is determined by a thorough evaluation that includes the following steps:
A focused radiation exposure history can be a critical tool for identifying dose-related signs and symptoms of radiation exposure and establishing triage priority.
Collecting the following information from patients can help providers develop a radiation dose estimate:
Information regarding the type and properties of the radioactive material involved in the incident should also be considered.
Basic radiation survey equipment in the hands of a qualified radiation professional can provide a great deal of information and can be used to initially direct medical care while awaiting results of specialized tests.
External contamination found on or near the nose or mouth, or over a wound, indicates the possibility of internal contamination via inhalation or ingestion.
Internal contamination may also be suggested when a survey finds a radiation hotspot over a part of the body that persists despite repeated attempts to decontaminate.

Figure 3: Ludlum Model 14c with pancake GM probe.
Source: REAC/TS
Laboratory testing is an important tool for:
However, in mass casualty settings the capacity for laboratory testing may be limited.
Table 2 provides examples of tests used to assess radiation exposure and internal contamination.
| Test/Procedure Type | Use |
|---|---|
| Urine or stool bioassay | Measures the type and amount of radioactivity in the sample |
| Whole body counters | Directly measures the amount of radioactivity in a person’s body |
| Serial lymphocyte counts, Cytogenetic biodosimetry | Help to quantify the radiation dose absorbed by the body |
Clinicians, with the help of radiation health professionals, will evaluate patient exposure history, radiation survey, and laboratory results to determine whether treatment with medical countermeasures is needed.
Clinicians can also use the Clinical Decision Guide (CDG) as a benchmark to determine whether to treat with medical countermeasures. The CDG is specific to the radionuclide and route of exposure (e.g., cesium-137 inhalational exposure) and, for most radionuclides, represents an effective dose of 0.25 Sieverts (Sv) after incorporation into the body. The CDG will be explained in more detail later in this module.
Normally, the presence of internal contamination - in excess of the CDG threshold and from a radionuclide that has an available countermeasure - is a strong indication for treatment.
However, during mass casualty emergencies, demand for treatment may overwhelm available resources. During times of resource scarcity, public health and medical officials may use a number of parameters to manage and direct those resources.
Table 3 provides examples of parameters to consider when triaging patients.
| Parameter | Indication |
|---|---|
| Prodromal Symptoms | Vomiting, nausea, or severe diarrhea within minutes of exposure, central nervous system (CNS) manifestations like loss of consciousness or coma, fever, and shock; these symptoms suggest a rapid, terminal prognosis, and therefore may be used to allocate medications to those with better chances of survival. |
| Injury Status | Reasons for potential triage to a lower priority:
|
| Laboratory Status | If over two days there is a 50% decrease in absolute lymphocyte count to less than 1000 cells per microliter of blood, it can be assumed that at least a moderate radiation dose was received. |
(Source: CDC)
Medical countermeasures that could be used during a radiation emergency include:
These will be discussed in greater depth in the following sections.
In addition to these four countermeasures, there are other supportive medications that will likely be needed. Careful planning for additional supplies in these categories may be required, especially in mass casualty incidents:
Video 4: Potassium Iodide (KI)
Read Transcript, Download (.mov, 46MB)
This section introduces potassium iodide (KI) and reviews its indications, administration, adverse effects, and some special considerations.
Afterwards, there is the option to apply what you have learned within a case study.
Figure 4: Potassium Iodide (KI)
Potassium iodide (KI) is a salt of stable, non-radioactive, iodine that acts as a blocking agent by directly competing against radioactive iodine for absorption by the thyroid.
Taking KI before or immediately after exposure to radioactive iodine protects the thyroid from radiation injury. Other body parts are not protected by the use of potassium iodide.
Only Food and Drug Administration-approved preparations of potassium iodide should be used. While these products are available without a prescription, their availability is not guaranteed and shortages may occur during periods of high demand.
Other iodine containing preparations - such as saturated solution of potassium iodide (SSKI) and Lugol’s solution - should not be substituted for FDA-approved preparations and are not recommended.
Use of KI should be considered shortly after exposure to radioactive iodine, or when the possibility of such exposure exists. Exposure to radioactive iodine is a concern following a nuclear power accident or the detonation of an improvised nuclear device (IND).
The efficacy of potassium iodide depends on the timing of administration:
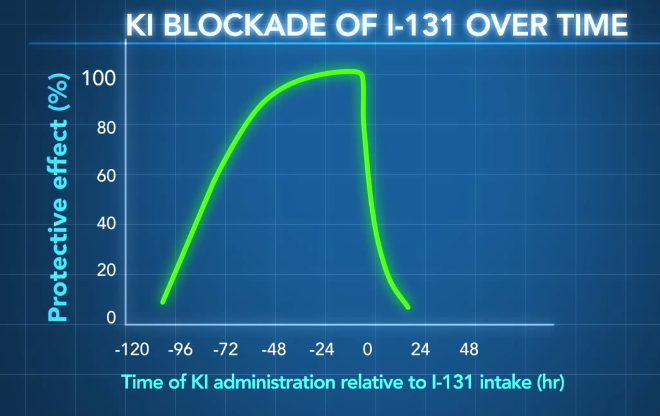
Figure 5: Time-dependence of KI efficacy. Concept only; Not for clinical reference. Citation: http://www.ncbi.nlm.nih.gov/pubmed/10832925
Table 4 provides information on cancer risk from radioactive iodine by age.
| Risk of Radioiodine Induced Thyroid Cancer | Population |
|---|---|
| Most Risk | Infants and children, as well as pregnant and nursing women |
| Moderate Risk | Adults 18–40 years old |
| Least Risk | Individuals over 40 years old |
All forms of KI are taken orally, either in tablet or liquid form. Tablets can be dissolved in liquid for easier administration.
Table 5 provides detailed recommendations on the exposure levels indicating KI use and the appropriate doses for different groups.
| Risk Group | Predicted Thyroid gland exposure (cGy) | KI dose (mg) | Number or fraction of 130 mg tablets | Number or fraction of 65 mg tablets | Milliliters (mL) of oral solution, 65 mg/mL*** |
|---|---|---|---|---|---|
| Adults over 40 years | ≥ 500 | 130 | 1 | 2 | 2 mL |
| Adults over 18 through 40 years | ≥ 10 | 130 | 1 | 2 | 2 mL |
| Pregnant or Lactating Women | ≥ 5 | 130 | 1 | 2 | 2 mL |
| Adolescents, 12 through 18 years* | ≥ 5 | 65 | 1/2 | 1 | 1 mL |
| Children over 3 years through 12 years | ≥ 5 | 65 | 1/2 | 1 | 1 mL |
| Children 1 month through 3 years | ≥ 5 | 32 | Use KI oral solution** | 1/2 | 0.5 mL |
| Infants birth through 1 month | ≥ 5 | 16 | Use KI oral solution** | Use KI oral solution** | 0.25 mL |
(Source: FDA)
* Adolescents approaching adult size (> 150 lbs) should receive the full adult dose (130 mg)
** Potassium iodide oral solution is supplied in 1 oz (30 mL) bottles with a dropper marked for 1, 0.5, and 0.25 mL dosing. each mL contains 65 mg potassium iodide.
*** See the Home Preparation Procedure for Emergency Administration of Potassium Iodide Tablets to Infants and Small Children.
It has been suggested that administering KI to young children is easier when flavored with raspberry, chocolate milk, orange juice, or flat soda.
Monitor patients for allergic and other adverse reactions to KI.
The adverse effects of KI use are more likely seen if a person:
Adverse effects may include:
Individuals may also experience the following symptoms of “iodism”:
Infants less than one month old who receive more than one dose of KI are at risk for developing hypothyroidism.
Contraindications to potassium iodide include (*Rare conditions):
Allergy to shellfish, povidone-iodine, or to radiocontrast media is not equivalent to iodine allergy.
Infants, pregnant, or breastfeeding individuals:
Adults over 40 years old:
More information can be found in the additional resources section below.
Video 5: Case Study - KI
Read Transcript, Download (.mov, 90MB)
Directions: You may choose to watch the video and follow the prompts or click on each scene summary button below and answer all questions.
Scene 1 Summary:

Figure 6: Chernobyl reactor
Scene 2 Summary:
Scene 3 Summary:
Scene 4 Summary:
Video 6: DTPA
Read Transcript, Download (.mov, 41MB)
This section introduces DTPA and discuss its indications, administration, adverse effects, and special considerations.
Afterwards, there is the option to apply what you have learned within a case study.
Diethylene triamine pentaacetic acid, or DTPA, accelerates renal elimination of radioactive materials from the body in urine and helps to reduce the duration of radiation exposure the body receives from:
DTPA does not repair or protect the body from radiation damage.
DTPA treatment may actually increase the deposition of uranium and neptunium into bone and thus is not recommended treatment for contamination with these radionuclides.
There are two available forms of DTPA used to treat internal contamination – calcium-DTPA (Ca-DTPA) and zinc-DTPA (Zn-DTPA).
Distribution of DTPA from state and federal stockpiles will be managed by public health agencies and administered by medical personnel.
Use of DTPA is indicated when individuals have been internally contaminated with a significant amount of radioactive plutonium, americium, and/or curium. To assist in determining need for DTPA:
Starting DTPA treatment as close to the time of exposure as possible is ideal, but even delayed administration can be useful.
Ca-DTPA and Zn-DTPA are indicated differently based on the patient’s time since radiation exposure:
Pregnant women should receive Zn-DTPA if available. However, for pregnant women and other populations, if Zn-DTPA is not available, long-term treatment with Ca-DTPA is still indicated but should be given with a multivitamin supplement and monitoring for mineral depletion, particularly zinc, magnesium, and manganese.
DTPA can be administered intravenously or by nebulizer. All forms of DTPA should be given as a single dose, once daily.
Tables 6 and 7 provide information on DTPA dosing and administration for adults and children.
| Route | Dose of DTPA |
|---|---|
| IV Push | 1g of DTPA solution, available as a single vial with a volume of 5 mL, can be administered via slow IV push over 3-4 minutes |
| IV Infusion | 1g of DTPA solution can be diluted in 100-250 mL of 5% Dextrose in Water, Ringers Lactate, or Normal Saline and administered over 30 minutes. |
| Nebulizer | 1g of DTPA solution, 1 gram in 5 mL, can be 1:1 diluted with 5mL sterile water or Normal Saline, and inhaled over 15-20 minutes NOTE: Both Zn-DTPA and Ca-DTPA may exacerbate asthma. |
(Source: REMM)
| Route | Dose of DTPA |
|---|---|
| IV Push | 14mg/kg/day, not to exceed 1g/day, of DTPA can be administered via slow IV push over 3-4 minutes |
| IV Infusion | 14mg/kg/day, not to exceed 1g/day, of DTPA can be diluted in 100-250 mL of 5% Dextrose in Water, Ringers Lactate, or Normal Saline and administered over 30 minutes |
(Source: REMM)
When possible, obtain samples of blood, urine, or feces to determine the amount of radioactivity present and to establish baseline laboratory values before initiating treatment.
Laboratory tests often used to monitor patient condition and treatment status include:
These samples should be routinely collected to establish the effectiveness of treatment and to determine when treatment can be terminated.
Individuals that receive DTPA should also be monitored for allergic and other adverse reactions.
The main adverse effects of DTPA use include:
Patients receiving nebulized DTPA may also experience breathing difficulties.
There are no known contraindications for DTPA treatment.
Ca-DTPA should be used cautiously with:
Nebulized DTPA treatment may be associated with exacerbations of asthma.
Video 7: Case Study: DTPA
Read Transcript, Download (.mov, 130MB)
Directions: You may choose to watch the video and follow the prompts or click on each scene summary button below and answer all questions.
Scene 1 Summary:

Figure 7: Truck accident with damaged cargo
Scene 2 Summary:
Scene 3 Summary:
Scene 4 Summary:
Video 8: Prussian Blue
Read Transcript, Download (.mov, 24MB)
This section introduces Prussian blue insoluble (referenced as Prussian blue in the rest of the module), and discusses its indications, administration, adverse effects, and special considerations.
Afterwards, there is the option to apply what you have learned within a case study.
Prussian blue reduces radiation exposure by binding to and enhancing elimination of radioactive cesium and thallium. These radionuclides are eliminated through the gastrointestinal tract, thereby preventing their reabsorption into the body.
Prussian blue is only effective for cesium and thallium. It does not work for other radionuclides and does not repair radiation damage that has already occurred.
Prussian blue comes in 500 mg blue capsules. It is a prescription medication that will be provided by public health officials or medical personnel after an emergency.
Prussian blue is currently kept in federal stockpiles for distribution as needed during a radiation emergency.
Prussian blue is indicated when individuals have been internally contaminated with a significant dose of radioactive cesium or thallium.
Prussian blue is FDA-approved for treatment of adults and children ages 2 and older. At this time, studies have not determined a safe dose for individuals younger than two years old; however, use in children under two may be authorized under an Emergency Use Authorization granted by the FDA at the time of an event.
To assist in determining need for Prussian blue:
Prussian blue is given orally, usually as a capsule. However, Prussian blue capsules may be opened and added to food or liquid for easier administration, or can be administered via nasogastric tubes, orogastric tubes, or any other device that can deliver the medication to the gastrointestinal tract.
Safe treatment with Prussian blue has not been established for individuals younger than two years old.
Table 8 provides information on Prussian blue dosing and administration for adults and children.
| Category | Route | Dose of Prussian Blue |
|---|---|---|
| Adults and adolescents (12+ yrs) | Oral | 3g, three times per day |
| Children (2-12 yrs) | Oral | 1g, three times per day |
(Source: FDA label)
Treatment with Prussian blue is recommended for a minimum of 30 days.
Treatment effectiveness and decisions on discontinuation are determined by periodic whole body radiation counts and monitoring of radioactivity levels in urine and stool samples.
Prussian blue is known to cause hypokalemia and therefore:
During the course of Prussian blue treatment, samples of blood, urine, or feces may be collected to establish the effectiveness of treatment and/or the amount of radioactivity present.
Individuals that receive Prussian blue should also be monitored for allergic and other adverse reactions.
There are no known contraindications for Prussian blue treatment.
Prussian blue artist’s dye is not the same chemical as Prussian blue insoluble and should not be taken in an effort to treat internal contamination.
Only FDA-approved forms of Prussian blue should be used.
Video 9: Case Study - Prussian Blue
Read Transcript, Download (.mov, 151MB)
Directions: You may choose to watch the video and follow the prompts or click on each scene summary button below and answer all questions.
Scene 1 Summary:

Figure 8: Screening for radiation in Goiania, Brazil (Source: NCEC)
Scene 2 Summary:
Scene 3 Summary:
Scene 4 Summary:
Video 10: Colony Stimulating Factors
Read Transcript, Download (.mov, 28MB)
This section introduces colony stimulating factors and discusses its indications, administration, adverse effects, and special considerations.
Afterwards, there is the option to apply what you have learned within a case study.
Colony stimulating factors (CSFs) are used to treat bone marrow suppression by stimulating the development of granulocytes – in particular, neutrophils. Colony stimulating factors are commonly used for bone marrow suppression in patients undergoing chemotherapy.
The effect of treatment is to help reestablish and support the body’s ability to fight infection and produce white blood cells.
It is anticipated that these medications will be recommended for use during a radiation emergency under an Emergency Use Authorization (EUA) from the FDA related to the treatment of acute radiation syndrome.
Colony stimulating factors are currently part of federal stockpiles.
Hospital supplies of colony stimulating factors may be depleted quickly during a mass casualty event, and public health officials will need to manage and direct federal resources to maximize their benefit during a radiation emergency.
The decision to treat a patient with colony stimulating factors should only be made after a careful assessment of risk by a team of knowledgeable professionals that include:
Table 9 describes indications that should be considered when determining the need for colony stimulating factors.
| Potential Indications for Treatment with CSFs | Details |
|---|---|
| Symptomology | Rapid onset of moderate or severe prodromal symptoms of acute radiation syndrome:
|
| Radiation Dose | Greater than or equal to 2 Gy whole or (significant) partial body exposure |
| White Blood Cell Counts | A high probability of prolonged, significant neutropenia. |
Early treatment with colony stimulating factors, particularly within the first 72 hours after exposure, is thought to provide more benefit than delayed treatment. However, even initiating treatment several days after exposure may be beneficial.
In general, treatment should continue until an individual’s absolute neutrophil count is consistently above 1000 cells per microliter of blood.
Colony stimulating factors are generally administered subcutaneously (SC), but may also be given intravenously (IV).
Table 10 provides dosing and administration information for three different colony stimulating factors.
| Category | Example | Adult Dose |
|---|---|---|
| G-CSF | filgrastim |
|
| Pegylated G-CSF | pegfilgrastim |
|
| GM-CSF | sargramostim |
|
(Source: REMM)
G-CSF = granulocyte colony-stimulating factor
GM-CSF = granulocyte-macrophage colony-stimulating factor
Individuals that receive colony stimulating factors should be monitored for:
The adverse effects of colony stimulating factors include:
Other severe adverse effects associated with use of colony stimulating factors include:
Colony stimulating factors should not be administered to individuals with a known history of hypersensitivity reactions to CSF medications or to E. coli-derived proteins.
Excretion of CSF components into breast milk is not known at this time.
Video 11: Case Study - Colony Stimulating Factors (CSFs)
Read Transcript, Download (.mov, 59MB)
Directions: You may choose to watch the video and follow the prompts or click on each scene summary button below and answer all questions.
Scene 1 Summary:
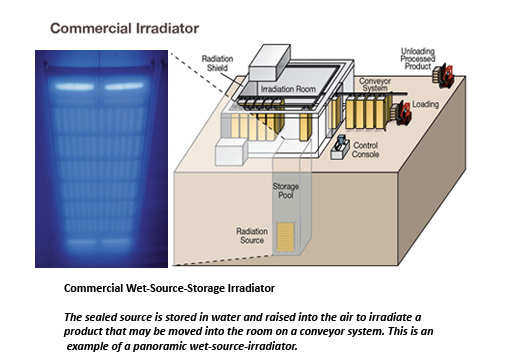
Figure 9: Commercial Irradiator (Source: Adapted from NRC, photo courtesy of Nordion.)
Scene 2 Summary:
Scene 3 Summary:
Scene 4 Summary:
Video 12: Clinical Decision Guides
Read Transcript, Download (.mov, 14MB)
This final segment introduces the Clinical Decision Guide, a critical benchmark that can be used by clinicians in addition to other factors to help determine if medical countermeasures are indicated for a particular patient.
The Clinical Decision Guide (CDG) is an operational quantity that is specific to a type of radionuclide and a route of exposure (e.g. cesium-137 inhalational exposure). The CDG of children (0-18 yrs) and pregnant women is 20% that of adults.
For most radionuclides, the CDG is defined by the amount (activity) of radioactive material delivering an effective dose of 0.25 Sieverts (Sv) after incorporation into the body. This dose represents about a 1.3% lifetime risk of fatal cancer attributable to the exposure. Radiation measurements from a urine sample above a CDG value suggest the potential for a higher attributable lifetime fatal cancer risk and may help clinicians decide whether or not to start countermeasure treatment.
The CDG can also be used as a screening level indicating the need for a more comprehensive investigation of the radiation dose received by a patient.
More information about the Clinical Decision Guide can be found in the National Council on Radiation Protection and Measurements report number 161 (NRCP 161).
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2013, 8 5). CDC Radiation Emergencies | Treatments for Radiation Exposure and Contamination. Retrieved 1 26, 2014, from http://www.emergency.cdc.gov/radiation/countermeasures.asp
FDA. (2009, 4 7). Bioterrorism and Drug Preparedness – Questions and Answers on Calcium-DTPA and Zinc-DTPA (Updated). Retrieved 2 5, 2014, from http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130314.htm
NLM – REMM. (2013, 8 30). Potassium Iodide (KI) – Radiation Emergency Medical Management. Retrieved 2 10, 2014, from http://www.remm.nlm.gov/potassiumiodide.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2006, 4 15). Medical Response to Mass Casualties – Planning. Retrieved 01 23, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Planning.htm
CDC. (2006, 4 15). Medical Response to Mass Casualties – Treatment. Retrieved 1 26, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Treatment.htm
CDC. (2006, 4 15). Medical Response to Mass Casualties – Triage. Retrieved 1 26, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Triage.htm
NCRP, N. C. (2008). Report No. 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008). http://www.ncrppublications.org/reports/161_i. Retrieved from 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008): http://www.ncrppublications.org/reports/161_i
NLM - REMM. (2013, 8 30). Potassium Iodide (KI) – Radiation Emergency Medical Management. Retrieved 2 10, 2014, from http://www.remm.nlm.gov/potassiumiodide.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2011, 3 18). CDC Radiation Emergencies | Radiation and Potassium Iodide (KI). Retrieved 2 3, 2014, from http://emergency.cdc.gov/radiation/japan/ki.asp
CDC. (2013, 8 5). CDC Radiation Emergencies | Treatments for Radiation Exposure and Contamination. Retrieved 1 26, 2014, from http://www.emergency.cdc.gov/radiation/countermeasures.asp
FDA. (2011, 5 4). Bioterrorism and Drug Preparedness – Frequently Asked Questions on Potassium Iodide (KI). Retrieved 2 4, 2014, from http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm072265.htm
NLM – REMM. (2013, 8 30). Potassium Iodide (KI) – Radiation Emergency Medical Management. Retrieved 2 10, 2014, from http://www.remm.nlm.gov/potassiumiodide.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2013, 8 5). CDC Radiation Emergencies | Treatments for Radiation Exposure and Contamination. Retrieved 1 26, 2014, from http://www.emergency.cdc.gov/radiation/countermeasures.asp
FDA. (2009, 4 7). Bioterrorism and Drug Preparedness – Questions and Answers on Calcium-DTPA and Zinc-DTPA (Updated). Retrieved 2 5, 2014, from http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130314.htm
NCRP, N. C. (2008). Report No. 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008). http://www.ncrppublications.org/reports/161_i. Retrieved from 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008): http://www.ncrppublications.org/reports/161_i
NLM – REMM. (2013, 8 30). Ca-DTPA/Zn-DTPA (Diethylentriamene pentaacetate) – Radiation Emergency Medical Management. Retrieved 2 11, 2014, from http://www.remm.nlm.gov/dtpa.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2013, 8 5). CDC Radiation Emergencies | Treatments for Radiation Exposure and Contamination. Retrieved 1 26, 2014, from http://www.emergency.cdc.gov/radiation/countermeasures.asp
FDA. (2003, 2 3). Guidance for Industry on Prussian Blue for Treatment of Internal Contamination With Thallium or Radioactive Cesium; Availability. Retrieved 2 5, 2014, from http://www.fda.gov/OHRMS/DOCKETS/98fr/03-2597.htm
FDA. (2009, 4 7). Bioterrorism and Drug Preparedness – Questions and Answers on Prussian Blue. Retrieved 2 5, 2014, from http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130337.htm
NCRP, N. C. (2008). Report No. 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008). http://www.ncrppublications.org/reports/161_i. Retrieved from 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008): http://www.ncrppublications.org/reports/161_i
NLM – REMM. (2013, 8 30). Prussian Blue – Radiation Emergency Medical Management. Retrieved 2 12, 2014, from http://www.remm.nlm.gov/prussianblue.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
Amgen. (2013, 9). NEUPOGEN (filgrastim). Retrieved 2 10, 2014, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103353s5157lbl.pdf
CDC. (2006, 4 15). Medical Response to Mass Casualties – Pharmacotherapy. Retrieved 1 21, 2014, from http://www.orau.gov/hsc/RadMassCasualties/content/Pharmacotherapy.htm
CDC. (2013, 8 5). CDC Radiation Emergencies | Treatments for Radiation Exposure and Contamination. Retrieved 1 26, 2014, from http://www.emergency.cdc.gov/radiation/countermeasures.asp
CDC. (2013, 11 1). Safety Information – Neupogen (filgrastim). Retrieved 2 10, 2014, from http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm219032.htm
NLM – REMM. (2013, 8 30). White Cell Growth Factors/Cytokines – Radiation Emergency Medical Management. Retrieved 2 11, 2014, from http://www.remm.nlm.gov/cytokines.htm
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
NCRP, N. C. (2008). Report No. 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008). http://www.ncrppublications.org/reports/161_i. Retrieved from 161 I – Management of Persons Contaminated With Radionuclides: Handbook (2008): http://www.ncrppublications.org/reports/161_i
REAC/TS. (2013). The Medical Aspects of Radiation Incidents. Oak Ridge: REAC/TS.
This training was prepared for Centers for Disease Control and Prevention by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement with the U.S. Department of Energy (DOE). ORISE is managed by Oak Ridge Associated Universities under DOE contract number DE-AC05-06OR23100.